MEDICAL DEVICE MANUFACTURING
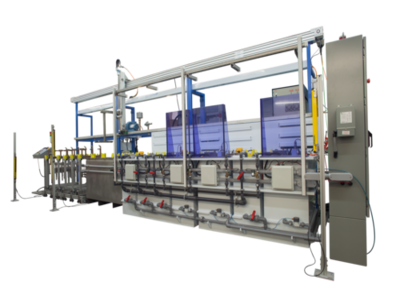
Manual and Automatic Passivation and Electropolishing Lines include process rinse water recycling for enhanced process control, quality and waste minimization.
Forward Thinking Water Management and Metal Finishing Systems for Medical Device Manufacturing
Clean Water
Due to ever mounting external mandates regarding clean water optimization, combined with the need to continually improve product quality in this competitive industry; Medical Device manufacturers require the creative strategic partnership that AQUASGROUP can provide.
AQUASGROUP is a chemical engineering based design/build manufacturer and service provider of custom automated Zero Liquid Discharge (ZLD) metal finishing lines, including systems for both electropolishing and passivation. Our leadership in design incorporates automation, closed loop rinse water and waste management, along with enhanced process control, all in one custom solution.

Effective solutions for Medical clean manufacturing:

Hollow Fiber Ultrafiltration Membrane Module
AG GFS FPI-UF/HF Green Factory Series® Hollow Fiber Ultra Filtration Membrane Module for emulsified oil / water separation. Applications include FPI rinse water, machine tool coolant and compressor condensate.

Process Water Re-Purification System Using RO and ION Exchange Technology
AG GFS BL1000 Process Water Re-Purification System Using RO and ION Exchange Technology
Mailing Address:
Corporate Headquarters & Fabrication Division
41 Commercial Way
East Providence, RI 02914
Water Technologies & Service Division
830 Waterman Avenue
East Providence, RI 02914